
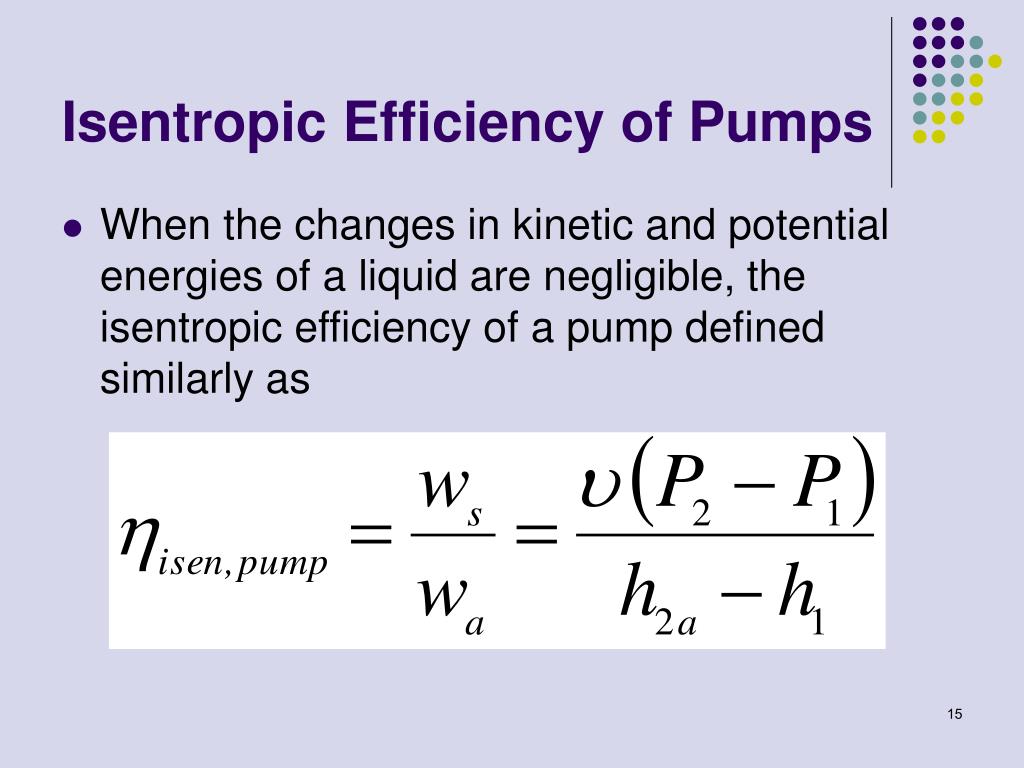
For example, a PSV or pressure reduction valve in a gas system, depending of the fluid properties and pressure drop at the restriction, during the depressurizing process the valve body may reach a very low temperature. The isentropic process leads to a decreasing in the fluid temperature. Therefore, the final process is isenthalpic.Įven so, the isentropic process occurs during a short period of time, it must not be ignored. The heat drop that caused the initial increase in kinetic energy is reclaimed (except for a small portion lost due to the effects of friction). As the valve is opened further, mass flow rate m increases, but the overall behavior of the pressure is the same, as seen in curve (B). Mass flow rate m is very small under these conditions, as seen in Figure 1c.
This fall in velocity requires a reduction in kinetic energy which is mostly re-converted back into heat and re-absorbed by the steam. decrease through the converging portion of the nozzle, and then increases through the diverging nozzle. 8.2 General Forms of Nozzle Passages A nozzle is an element whose primary function is to convert enthalpy (total. The nozzle is so shaped that it will perform this conversion of energy with minimum loss. Having passed through the narrow restriction, the steam expands into the lower pressure region in the valve outlet, and eventually decelerates as the volume of the valve body increases to connect to the downstream pipe. The increase of velocity of the steam jet at the exit of the nozzle is obtained due to decrease in enthalpy (total heat content) of the steam. It does so by borrowing energy from its enthalpy and converting it to kinetic energy. For the steam to squeeze through the narrow restriction it has to accelerate to a higher speed. Using chlorophyll in the process called photosynthesis, they convert the suns energy into storable form in ordered sugarmolecules. Using as example the steam expansion through a control valve orifice. The leaves use the energy from the sun in tiny energy factories called chloroplasts. However, the isentropic process occurs during a short period of time. In a throttling process (control valve, nozzle or orifice) overall process is isenthalpic.


 0 kommentar(er)
0 kommentar(er)
